Knowledge section / Edition 1 / 2015
Blue Energy promising for the water chain
Blue Energy, energy generated by mixing fresh and saltwater, has been well reported. At the end of last year, King Willem Alexander opened a pilot plant on the Afsluitdyke. Great interest is shown from abroad as well. Blue Energy: how does it work exactly, what are the possible applications and what is interesting for the water sector?
During the process of fresh water flowing into the sea, within moments a spontaneous mixing of fresh and salt water occurs. Saltwater contains a relatively large amount of charged particles (mainly Na- and Cl-ions). When freshwater and saltwater come into contact, the ions of the saltwater will move towards the freshwater. The instigator of this mixing process is the increase in entropy, in popular terms: the disorder of the system. The saltwater will gradually become more fresh and the freshwater more saline.
When this mixing process is occurring in an uncontrolled way – like in nature, or at a discharge – it is irreversible. By controlling the mixing process in a reversible way, a part of the energy that is otherwise lost, can be converted into electricity. In the Netherlands this has become known as Blue Energy. From mixing 1 cubic metre of freshwater per second with an equal amount of saltwater, theoretically and electrical power of 1.5 Megawatt can be produced. The energy density is comparable to a hydropower reservoir with a height of 150 metres.
From 2005, research institute Wetsus has gathered a consortium of universities, technology companies and end users together for research and development of the Blue Energy technology. The technology that was selected is called RED, Reverse Electro Dialysis. This process makes use of ion-selective membranes along which saltwater and freshwater are flowing. These alternately arranged membranes pass either positive or negative ions from the saltwater to the freshwater, creating a charge separation resulting in a voltage. Per membrane this voltage is circa 80 millivolt, enabling a stack of about 1,200 membranes to obtain 100 Volt.
The membrane stack is placed between an anode and a cathode in order to convert the ionic current into an electrical current. In this way, mixing energy is transformed into electrical energy. Both the membranes and the electrodes are key components, that have been developed further during recent years.
Research
Researchers’ first concern was to raise the power density – the power obtained per membrane area. From former literature it appeared that a lot of modelling with promising numbers had taken place, but the highest really measured power density was not higher than 0.41 Watt per square metre of membrane. In the past years the power density increased with a factor 5 when working with pure NaCl-solutions.
The next step was testing with artificial river and seawater: salt solutions with the same ion-composition as the natural fresh and salt water. It appeared that the density was reduced considerably with these ionic compositions, which was primarily attributed to other ions in the seawater (mainly the Mg-, Ca- and SO₄-ions). It was quite a surprise, however, to find that the composition of the freshwater had a large impact on the power density. While the Na- and Cl-ions were transported from saltwater to freshwater, the other ions present in the freshwater are actually transported in opposite direction. After this problem had been recognised, membranes were developed that are selective to single charged ions, reducing this contra productive process of ion exchange significantly.
Membrane development has been an important aspect of the research carried out by the University of Twente, necessary for decrease of the system’s internal electric resistance but also the hydraulic resistance and the fouling sensitivity. Membranes were developed with modified surfaces on which biofilms attach less quickly. Also membranes were developed with a profiled surface, to achieve optimal distribution, flow and mixing of the freshwater and saltwater. Besides, effort was made to decrease biofouling by varieties in operational measures.
A focus during the research phase was on the performance of the RED-technology, both in laboratory as in practice at the harbour and the salt factory of Esco in Harlingen. Based on these results the process design has been frequently adjusted. Now the time has come to practically test the developed technology in a large scale pilot and in long-term tests. The company REDstack together with Fujifilm, Magneto and several other companies connected to Wetsus – supported by the Province of Friesland and in close cooperation with Rijkswaterstaat (Dutch Department of Waterways and Public Works) – have realised a pilot installation on the Afsluitdyke. The pilot installation can mix 200 cubic metres of fresh lakewater from the IJsselmeer per hour with 200 cubic metres seawater from the Waddensea per hour. The pilot installation will be operational until the end of 2015.
Future large-scale applications will decrease the ecological impact of nowadays freshwater discharges by sluices at low tides as the freshwater in Blue Energy is pre-mixed with saltwater distributed over the whole day.
Freshwater and saltwater are primarily sieved and pumped through an installation. This takes place with a low flow rate. In this way, scavenging of larger organisms can easily be prevented.
Micro-organisms and small particles, however, will pass through the microsieves and will be carried along the membranes. Not only the effects of these particles on the achievement of the installation (fouling and growth on the membranes) but also the effects of the installation on these micro-organisms are part of the pilot research on the Afsluitdyke. A monitoring programme has been set up for measuring the impacts of the installation on the natural values.
Applications
The most obvious application of Blue Energy is the application as been described before, being implemented in the natural hydrological circle. For instance in places were freshwater is discharged or set out to sea, so at sea sluices or pumping stations. In the domestic and industrial water cycle the RED-technology can be applied when effluent is discharged in a receiving saline water body. Accordingly, water boards with wastewater treatment plants at sea can not only produce energy from the organic fraction in waste water, which is already been done, but in addition they can produce a comparable amount of electrical energy end-of-pipe.
Besides these applications there are less obvious possibilities in the water chain, for instance in dry areas in the world, and also for example on islands, being part of a desalination scheme. Saltwater desalination has a higher social acceptance than high value water re-use, but due to high energy use it is much less sustainable. Still it is possible to combine the best of both options - low energy use and the acceptance of seawater as a drinking water source - by adapting RED.
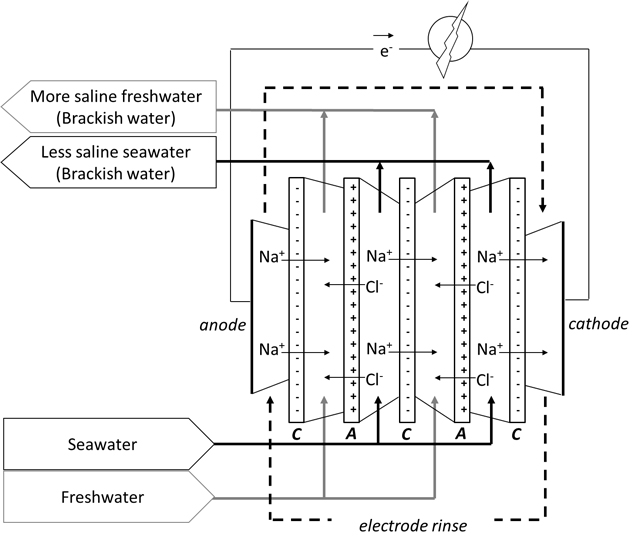 Schematic representation of a Reverse Electro Dialysis (RED) stack for conversion of mixing energy in electrical energy (Blue Energy): C is a negatively charged cation exchange membrane, A is a positively charged anion exchange membrane.
Schematic representation of a Reverse Electro Dialysis (RED) stack for conversion of mixing energy in electrical energy (Blue Energy): C is a negatively charged cation exchange membrane, A is a positively charged anion exchange membrane.
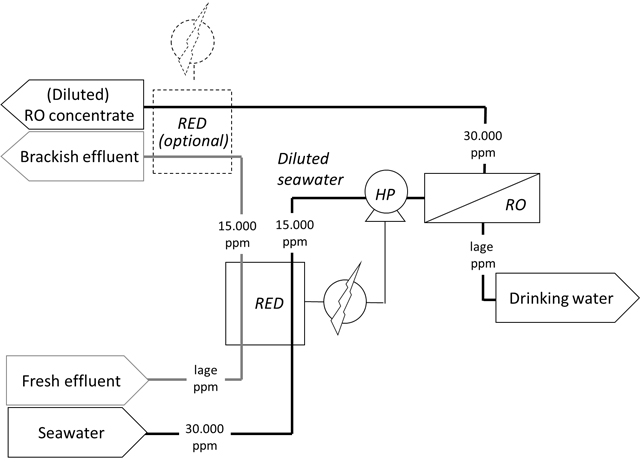 Schematic representation of Reverse Electro Dialysis (RED) applied as salt exchanger where salts from relatively clean seawater are transported to effluent as pre-treatment of a desalination installation. The numbers are indicative for salt content in ppm, RO for Reverse Osmosis and HD for High Pressure pump.
Schematic representation of Reverse Electro Dialysis (RED) applied as salt exchanger where salts from relatively clean seawater are transported to effluent as pre-treatment of a desalination installation. The numbers are indicative for salt content in ppm, RO for Reverse Osmosis and HD for High Pressure pump.
The most simple way to decrease energy use of seawater desalination is to lower the salt concentration. Sometimes this is already done by taking in pre-diluted seawater near an effluent discharge.
Energetically (and regarding acceptation), however, it is much better to have this pre-dilution occurring by controlled migration of salt from seawater to fresh water, as in RED. With RED-technology as a ‘salt exchanger ’, salt is transported from seawater to effluent. The saltwater is not directly in contact with the effluent, so contamination with pathogens cannot occur from the effluent to the seawater that is a source for drinking water production.
For the water supply of islands, desalination can become a sustainable option with a higher social acceptance than direct re-use of effluent or other polluted waste water streams. The lower concentration of salt provides energy saving for the following desalination step with reverse osmosis (RO) and the thus generated energy can also immediately be applied to the RO pump. In principle it is possible to produce an energetic self-sufficient desalination system. The reduction of the salt concentrations at the end additionally diminishes the amount of problems regarding concentrate discharge.
Obviously this needs more research. For instance on the issue whether and how heavy metals and organic micro pollutants in this system would be transported from the wastewater to the seawater and if they would be detectable in the finally prepared drinking water after RO.
In scientific articles many other applications of RED – or related CapMix technology which is also developed at Wetsus on the basis of capacitive electrodes – are referred to for possible use in the water chain. Combinations with microbial fuel cells are described, where waste water treatment and electricity production could take place in one step.
Also concepts are mentioned with RED as application for conversion of heat to electricity by a synthetic salt gradient, or in which gas emissions are transformed into electricity through a salt gradient. Additionally the development of an energy storage system based on salt gradients with a central role for RED or CapMix is in progress.
Jan Post
Wetsus/AquaBattery
Joost Veerman
REDstack
Rik Siebers
REDstack
Summary
Blue Energy is promising for water boards situated at sea. With this technique of controlled mixing of freshwater and saltwater they can produce energy in amounts comparable to those collected from the organic fraction in waste water. During an extensive research phase, particular focus has been on the operation of the RED-technology, in the laboratory as well as at the harbour and in the salt factory of Esco in Harlingen. At the Afsluitdyke a pilot installation that can mix 200 cubic metres lakewater from the IJsselmeer with 200 cubic metre of seawater from the Waddenzee per hour will be operational until the end of 2015. Apart from energy production less obvious possibilities of Blue Energy in the water chain are indicated, for instance as desalination technology.
Literature
J.W. Post (Wageningen University, 2009)
P. Dtugotęcki (University Twente, 2009)
J. Veerman (Rijksuniversity Groningen, 2010)
B.B. Sales (Wageningen University, 2013)
D.A. Vermaas (University Twente, 2014)
E. Güler (University Twente, 2014)
Li, W., W.B. Krantz, E.R. Cornelissen, J.W. Post, A.R.D. Verliefde, C.Y. Tang (2013), Applied Energy, 104, 592-602.
Auteurs

Jan Post
(Wetsus/AquaBattery)

Joost Veerman
(REDstack)

Rik Siebers
(REDstack)




