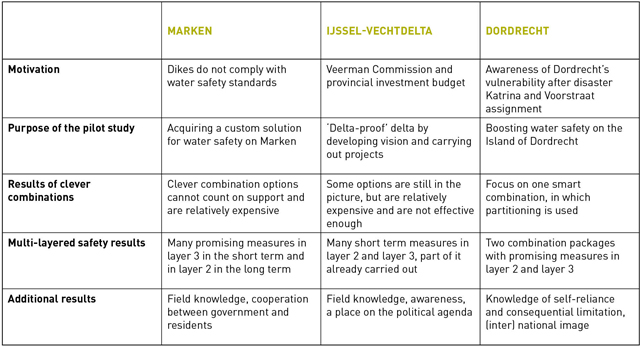
Removing drugs from wastewater: is it practically feasible?
Sewage treatment plants can only remove drugs partially from wastewater. Purification techniques for drinking water are able to do so, but are less suitable for the effluent of sewage treatment plants. It contains a lot of organic material. Is it possible and cost effective to remove this first and to then subject the wastewater to an advanced oxidation process?
The use of drugs is increasing sharply, in part because of an ageing population. A large part of these substances (and their metabolites) enter the sewer water via urine and faeces. It is expected that European standards will be adopted in the short or medium term on the amount of drugs that may be discharged. The placement of four substances on the EU watch list is a forerunner.
Nevertheless, a problem threatens. The current sewage treatment plants remove about 60 to 70 per cent of the remains of medicines (Figure 1). However, because more and more is used, the surface water will be more and more contaminated. This is not only a problem for the aquatic environment, but also for the production of drinking water.
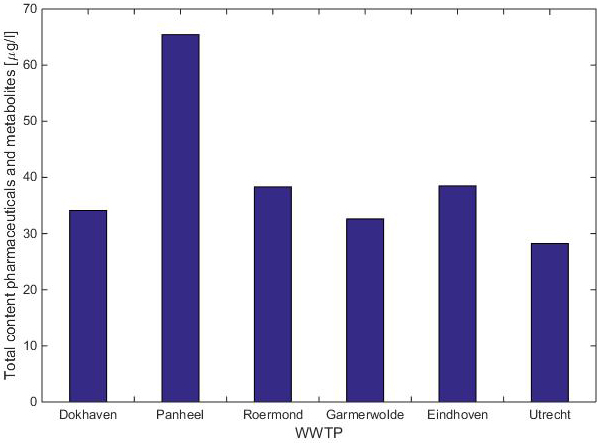
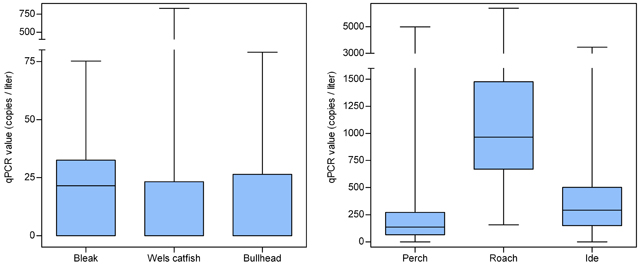
Figure 1. Total count of drugs and their metabolites in the effluent of the Panheel sewage treatment plant
Various techniques have been developed to purify drinking water in recent years, but it would actually be more effective to remove such substances at the source (think of households and hospitals). An end-of-pipe solution after the sewage treatment is also an option: good for the environment and also beneficial to the drinking water production.
In principle, techniques like advanced oxidation, which is used in drinking water production, could also be suitable for the removal or conversion of medicines in waste water.
The problem, however, is that the residual water (effluent) of sewage treatment plants contains a lot of Effluent Organic Material (EfOM). As the structure of this material corresponds somewhat with that of medicines, the EfOM disturbs in the decomposition process. It competes with the medicines for adsorption positions in adsorption processes, for example with activated carbon. As a result, the removal from effluent becomes relatively inefficient. In addition, harmful by-products could be created by reactions of the EfOM.
The project that is described in this article, examined whether it will be technically and possibly also economically more feasible if EfOM is removed (partly) via a separate pre-treatment. For this purpose, experiments were first performed on laboratory scale with two different pre-treatment methods and several advanced oxidation processes (AOPs) as the next step. On this basis, a pilot plant was built at a sewage treatment plant, where research takes place on a larger scale.
Effluent pre-treatment
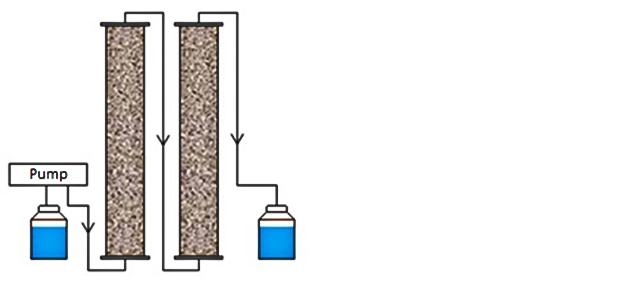
The research was carried out with the effluent of the Panheel sewage treatment plant, in which relatively high concentrations of medicines occur. Here, two different types of pre-treatments were applied:
• Ion Exchange (IEX): the water is filtered through a column with a resin that can filter negatively charged ions from the water.
• Ozone/bio-filtration (O3/bio-filtration): treatment with ozone allows for partial oxidation of certain compounds, which can then be broken down further using micro organisms.
The EfOM consists of various organic material groups, and both pre-treatment techniques have a different influence on these groups (table 1).
Table 1
Various components in EfOM (mm = molar mass)
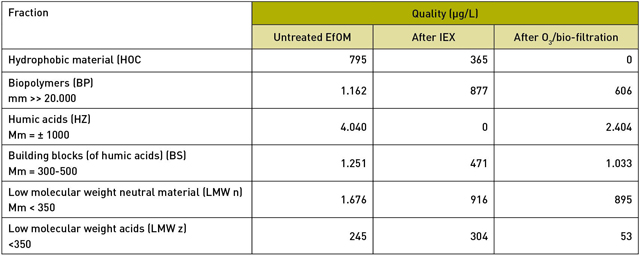
IEX appears to remove all the humic acids from the effluent. In addition, this treatment technique removes about half of the hydrophobic material, 60 perc ent of the building blocks and a quarter of the biopolymers.
O
3/biofiltration has a very different effect. This technique removes all hydrophobic material, about half of the biopolymers, 40 per cent of the humic acids and about 20 per cent of the building blocks. So, a big difference.
Advanced oxidation as next step
Advanced oxidation (AOP) uses highly reactive hydroxyl radicals, able to break down a wide range of organic compounds. For example, hydroxyl radicals are formed in a UV/H2O2 process. With this process medicines can be broken down in two different ways:
• Depending on the wavelength being used, some molecules can absorb UV radiation and then fall apart. This process is called photolysis.
• H2O2 can also absorb UV radiation and then break down into two hydroxyl radicals (• OH). These hydroxyl radicals can then oxidise many types of organic compounds.
UV radiation is often used to disinfect drinking water. An important parameter in UV processes is the amount of UV energy, also called the dose. Advanced oxidation requires about ten times as high a dose than that which is needed for disinfection. In addition, the water of a sewage treatment plant is badly permeable to UV radiation, rendering the UV process very ineffective.
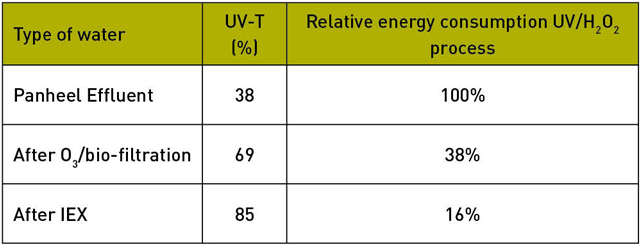
By removing (part of) the EfOM from the effluent, the water becomes more permeable to UV radiation, as a result of which the energy consumption of the UV process is greatly reduced (table 2).
Table 2
The effect of different pre-treatment on UV-T and energy consumption
Initially, a mixture of more than 30 medicines and some control substances like caffeine were added to the Panheel effluent, and the effect of the different treatments on the effectiveness of a subsequent advanced oxidation process was studied on a laboratory scale. Not only the conversion of the added micro-pollutants was looked at, but also the degradation and formation of some (known) metabolites of drugs (which, incidentally, were not dosed).
The investigation indicated that after a pre-treatment using IEX at a dose of 300 milljoule (mJ) per square centimetre, most medicines were already degraded to concentrations below the cut-off. In comparison: an advanced oxidation process usually needs at least 500 millijoule per square centimetre.
This means the UV/H
2O
2 process is very efficient at applying a pre-treatment with, for example, IEX, not only because a low UV dose suffices, but also because relatively little energy is required to reach that dose (see table 2).
In addition to the above oxidation process based on UV/H
2O
2, experiments were also performed in the laboratory with other oxidation processes, such as O
3/H
2O
2 and O
3/UV.
On the basis of the results, it was decided to conduct a pilot experiment on the Panheel sewage treatment plant, in which both pre-treatment processes would be studied side by side, each followed by a UV/H
2O
2 process and an activated carbon filter. This filter is intended primarily to eliminate the excess H
2O
2, but also as an additional barrier, because, after all, a relatively high concentration of medicines was dosed in the research. Here, too, a similar improvement of the UV-T, and thus of the UV/H
2O
2 process, was found throughout the duration of the experiment (approximately three months).
Optimising the purification process
The experiments' results indicated that the removal of part of the EfOM did in fact lead to a much more efficient UV/H2O2 process on a larger scale, with a significantly lower energy consumption. This effect is the greatest with a pre-treatment with IEX, but that is countered by the fact that its application generates a concentrated waste stream with a high salt concentration, which may probably not just be discharged, and that may also still contain traces of drugs. Extra costs will have to be incurred for the processing of this concentrate. The pre-treatment with O3/bio filtration doesn't have this disadvantage.
Furthermore, the investigation showed that metabolites can also be properly converted in the tested processes, and that a higher conversion is generally found after pre-treatment. At the same time, this shows that additional metabolites are formed in some cases during the oxidation process. In applying a lower dose, the original medicines are sufficiently converted, but other undesirable products, such as carbamazepine-10,11-epoxide, may be formed.
This is also a trade off that should be taken into account in a decision to implement such a process widely. What dose delivers an acceptable result at the lowest possible cost? This is a policy question for the District Water Boards. Because the current analysis techniques are so good, there is always something that can be shown, the question of which low levels are acceptable has more to do with politics and imaging than with risks for the environment and public health.
Of course, the expected cost plays a large role in any optimisation. A report by the Foundation for Applied Water Research (STOWA) indicates that ozonisation in combination with downstream sand filtration would cost approximately 0.2 to 0.3 Euro per cubic meter. Using the CoP cost Calculator by Royal HaskoningDHV, a first indicative estimate was made of the additional costs that the pilot set-up processes would incur. This estimate shows that the costs for these processes are about the same as the above mentioned processes. It still didn't take into account the increased efficiency of the UV/H2O2 process, generating a considerable saving on energy consumption and possible chemicals that may be necessary for this process.
Conclusions
• The effluent of sewage treatment plants still contains significant quantities of drugs and their metabolites.
• With advanced oxidation processes such as UV/H2O2, these organic micro pollutants can be broken down efficiently if the EfOM is (partly) removed first.
The two tested pre-treatment techniques are both suitable. Pre-treatment with ion exchange generated the greatest energy gains for the process, but it generates a salty waste stream (with a part of the drugs), which will have to be treated afterwards. Pre-treatment with O3/bio filtration offers less energy savings, but also no concentrate, and also removes a part of the medicines. This technique, however, is slightly more sensitive to disturbances.
• Optimisation of the processes should also take the possible formation and conversion of degradation products/metabolites into account. In addition to technical optimisation, imaging and cost also play an important role.
• The additional costs for such a combination of the studied processes, appear to be of the same order as that listed in the STOWA report. Because pre-treatment significantly improves the water quality for an advanced oxidation process, extra cost savings may probably be possible here.
Roberta Hofman-Caris
(KWR Watercycle Research Institute)
Wolter Siegers
(KWR Watercycle Research Institute)
Kevin van de Merlen
(AWWS)
Ad de Man
(WBL)
Summary
Drugs ending up in a sewage treatment plant via the sewer water, are only partly broken off there. As a result, residue remains in the surface water. Techniques to purify drinking water are often less effective in wastewater because it contains a lot of organic material. By removing a large part of this first, it is possible to convert medicines much more effective by using advanced oxidation processes. Although two purification processes are then required, this can be a cost saving compared to previous estimates, making treatment of waste water not only technically, but also economically more feasible. This was the result of a study at the Panheel sewage treatment plant in Limburg.
Literature
Mulder, M., Antakyali, D., Ante, S. (2015). Verwijdering van microverontreinigingen uit effluenten van RWZI’s; Een vertaling van kennis en ervaring uit Duitsland en Zwitserland. STOWA-rapport 2015-27.
Hofman, J., Laak, T.t., Tolkamp, H., Diepenbeek, P.v. (2013). Geneesmiddelen in de waterketen in Limburg: herkomst en effect. H2O-online 09-12-2013
Hofman, J., Tolkamp, H., Laak, T.t., Huiting, H., Hofman-Caris, R., Diepenbeek, P.v. (2013). Terugdringen van geneesmiddelen in de waterketen van Limburg. H2O-online 10-12-2013
^ Back to start